Managing a manufacturing business in the Philippines means constantly balancing tight production schedules, strict safety requirements, and the pressure to stay compliant. A single misstep in following Good Manufacturing Practices (GMP) can quickly turn into a costly setback.
Common issues like poor sanitation, incomplete records, or uncontrolled processes often slip through the cracks, undermining even the best GMP efforts. These gaps not only compromise product quality but also disrupt operations and erode customer trust.
Interestingly, research in the Philippines shows that while employees generally understand GMP standards, putting them into consistent practice is still a challenge. This highlights the urgent need for stronger systems and clearer processes to turn awareness into reliable day-to-day execution.
That’s why we invite you to explore practical strategies and smart software tools by HashMicro that can take your GMP management to the next level.
Table of Contents

Key Takeaways
|
What is GMP?
GMP stands for Good Manufacturing Practices, a system that ensures products such as food, cosmetics, and pharmaceuticals are consistently manufactured under strict quality standards. Applying GMP helps Philippine businesses avoid losses, recalls, and legal issues, protecting both the company and consumers from serious food safety risks.
GMP guidelines address every step of production to prevent problems like contamination, adulteration, and mislabeling. They cover crucial areas, including quality management, hygiene, facility upkeep, equipment, raw materials, and proper documentation.
Through regular inspections and GMP training, companies can uphold these standards and build trust in their products. Especially in the food industry, following GMP standards means safer operations, a stronger reputation, and compliance with both local and global requirements.
Difference Between GMP and cGMP
GMP stands for Good Manufacturing Practices, which are fundamental guidelines set by the FDA to ensure that manufacturers consistently produce safe and effective products. GMP centres on requiring businesses to take proactive steps to uphold quality across all operations.
Meanwhile, cGMP refers to current Good Manufacturing Practices, emphasizing continual improvement with modern systems and technologies to meet evolving quality standards while ensuring the Bill of Materials remains accurate and compliant.
For Philippine businesses, understanding this distinction is crucial. Investing in GMP training and staying aligned with cGMP principles helps ensure compliance, strengthens brand reputation, and promotes safer practices, especially vital in the food industry.
Main Components of Good Manufacturing Practices
Understanding these key elements is essential for any business aiming to implement GMP effectively and meet global standards. By focusing on these core components, Philippine manufacturers can build a strong foundation that ensures consistent quality, safety, and compliance throughout their operations.
- People: At the core of GMP, every employee is expected to follow manufacturing processes and regulations strictly. Regular GMP training ensures they fully understand their roles, boosting competency and compliance with good manufacturing practices.
- Products: All products must undergo rigorous testing, comparison, and quality checks before reaching consumers to ensure compliance with Good Manufacturing Practices guidelines. By defining clear specifications for raw materials and consistently applying standard methods, manufacturers secure reliable outcomes in the GMP food industry and beyond.
- Processes: Processes under GMP must be well-documented, clear, and consistently communicated to all staff. Ongoing evaluations help confirm that everyone adheres to current procedures and maintains the organization’s quality standards.
- Procedures: Procedures outline step-by-step instructions for critical tasks to achieve uniform results across production. Any deviations from these standard practices should be promptly reported and thoroughly investigated to maintain trust in GMP standards.
- Premises: Premises should always promote cleanliness and organization to prevent cross-contamination or accidents, which is vital in sectors like the food industry. Proper placement and regular calibration of equipment ensure consistent product quality and minimise risks associated with equipment failure.
10 Principles of GMP
Before you can fully comply with GMP guidelines, it’s crucial to understand the core principles that uphold these standards. These ten principles serve as a practical roadmap for businesses in the Philippines striving to implement good manufacturing practices effectively.
Below are 10 principles of good manufacturing practices:
-
Create Standard Operating Procedures (SOPs)
GMP stands for Good Manufacturing Practice, which emphasises consistent quality, starting with the establishment of clear Standard Operating Procedures (SOPs). These detailed instructions guide employees on exactly how to perform tasks in accordance with GMP guidelines.
Having robust SOPs minimizes errors and variability across the production line. This approach establishes a strong foundation for compliance, which is especially crucial in the
-
GMP food industry.
Enforce and implement SOPs and work instructions
It’s not enough to create SOPs; they must be actively enforced throughout your facility. Managers and supervisors should regularly observe and ensure that work instructions are followed precisely and accurately.
This helps maintain high standards aligned with good manufacturing practices. Over time, a culture of accountability takes root, safeguarding both product quality and consumer trust.
-
Document procedures and processes
Documentation is a cornerstone of GMP, meaning providing evidence that operations meet quality requirements. Keeping thorough records of every procedure and process supports traceability and simplifies audits.
It also helps quickly identify any deviations or non-conformities. Accurate documentation strengthens regulatory compliance and prepares your business for GMP audits.
-
Validate the effectiveness of SOPs
Validation confirms that your SOPs consistently produce the intended outcomes. Testing and reviewing processes ensure that methods are scientifically sound and reliable. If gaps or inefficiencies are found, they can be adjusted proactively.
-
Design and use working systems
Systems should be designed to support your workflow efficiently while minimizing risks. Whether it involves machinery layouts, manufacturing software, or quality controls, these must align with GMP guidelines.
Reliable systems reduce chances of contamination, errors, or unsafe conditions. Ultimately, they drive smoother operations and better product outcomes.
-
Maintain systems, facilities, and equipment
Regular maintenance of equipment and facilities is vital under good manufacturing practices. Clean, calibrated, and well-maintained assets ensure consistent production quality and reduce downtime.
Preventive maintenance also extends the life of critical systems. This proactive care safeguards both your investments and your brand reputation.
-
Develop the job competence of workers
People are integral to GMP success, making continuous good manufacturing practices training essential for maintaining this success. Employees should thoroughly understand their responsibilities and how their actions impact the overall quality. Investing in skill development boosts efficiency and reduces costly mistakes.
-
Prevent contamination through cleanliness
Cleanliness is a non-negotiable element in GMP, especially in food and pharmaceutical production. Strict sanitation prevents cross-contamination that could compromise safety. This includes cleaning equipment, maintaining hygienic facilities, and enforcing personal hygiene among staff.
-
Prioritize quality and integrate it into workflow
Quality shouldn’t just be a checkpoint—it must be woven into every stage of production. From receiving raw materials to packaging finished goods, each step should focus on meeting GMP standards.
Encouraging teams to prioritise quality promotes a shared sense of responsibility. This integrated approach minimises defects and strengthens long-term business performance.
-
Conduct GMP audits regularly
Regular audits verify that your operations align with GMP requirements and highlight areas for improvement. Internal and external inspections help catch issues before they escalate into costly recalls or legal troubles.
These audits also keep your teams sharp and continually engaged with GMP practices. By embracing audits as opportunities, Philippine businesses can build resilience and consumer confidence.
GMP Regulations
GMP regulations are legal requirements established by national governments to oversee the production, verification, and validation of products before they reach the market. These good manufacturing ERP practices ensure that every item is safe and effective, protecting consumers and upholding industry standards.
In the United States, good manufacturing practices stand for a strict system enforced by the FDA through cGMP guidelines, covering everything from food and cosmetics to medical devices and pharmaceuticals. Facility inspections are routinely conducted, and if serious violations are discovered, the FDA can recall products, resulting in significant losses and operational challenges for manufacturers.
The quality of manufactured goods is heavily regulated because lapses can harm both people and the environment. Poor hygiene, lack of temperature control, cross-contamination, or adulteration are just a few examples of risks that highlight why Good Manufacturing Practice (GMP) matters, especially in the food industry, and why regular GMP training and compliance are crucial.
GMP Standards
Understanding and applying GMP standards is essential for any Philippine business that wants to maintain high-quality products and protect consumers. These standards go beyond basic compliance; they build trust, strengthen your brand, and reduce costly mistakes.
By following key measures, you can embed good manufacturing practices into every part of your operations and secure long-term success.
-
Quality team
A dedicated quality team is crucial for any business aiming to uphold good manufacturing practices standards. These skilled professionals focus on refining existing manufacturing processes and ensuring strict compliance with good manufacturing practices.
They routinely assess operations, spot potential issues, and recommend corrective measures to protect both product quality and consumer safety. Scheduled monitoring of equipment, workflows, and staff competencies is also part of their essential role in sustaining GMP guidelines.
-
Validation
Validation is a documented process that proves instruments, methods, and activities consistently deliver expected results. Under the GMP meaning, this involves multiple areas such as process validation, cleaning protocols, computer systems, and analytical methods.
By validating these critical aspects, manufacturers reduce the risks of defects and ensure safer, higher-quality products. It also builds confidence among regulators and customers that your operations truly meet GMP standards.
-
Surprise audits
Conducting unannounced audits offers an honest snapshot of your facility’s compliance with GMP. These surprise checks help uncover root causes of non-compliance that might otherwise go unnoticed in scheduled inspections.
Addressing problems early prevents them from escalating into larger threats, such as costly recalls or damage to a reputation. This proactive approach reinforces your commitment to quality and safeguards the integrity of your brand.
-
Compliance training
Investing in GMP training equips your staff with the knowledge necessary to consistently uphold good manufacturing practices. Employees learn the importance of record-keeping, sanitation, equipment handling, labeling, and following SOPs, which collectively minimize human error.
Regular training also fosters a culture of quality and accountability within your organisation. Ultimately, well-informed teams are your strongest asset in maintaining continuous GMP compliance, especially critical in the food industry.
How to Comply with GMP Guidelines?
Before diving into production, it’s important to understand the core GMP guidelines that shape safe and consistent manufacturing practices:
- Building and facilities: Maintaining clean, well-designed facilities is a cornerstone of GMP guidelines, which directly impact product safety and quality. Proper layouts help control contamination and ensure smooth workflow, which is essential in the GMP food industry.
- Materials management: Effective handling and storage of raw materials prevent spoilage, mix-ups, and adulteration. This practice adheres to good manufacturing practices, ensuring consistent product quality throughout the entire production process.
- Quality control systems: Implementing rigorous quality checks throughout production guarantees products meet GMP standards. It also reduces defects, protects consumers, and reinforces your company’s reputation.
- Manufacturing: Every manufacturing step must align with GMP, meaning that it follows precise procedures and uses validated methods. This minimizes errors and ensures each product batch is safe and effective.
- Packaging and identification labeling: Clear labeling and secure packaging are critical under GMP guidelines to avoid misbranding and ensure traceability. These steps build trust and safeguard consumers from misuse.
- Quality management systems: A robust quality management system integrates good manufacturing practices into every process. It helps monitor compliance, drive improvements, and support long-term business success.
- Personnel and GMP training: Well-trained employees understand their role in maintaining GMP standards and are less likely to make costly mistakes. Regular GMP training builds skills, boosts morale, and ensures everyone works toward the same quality goals.
- Purchasing: Sourcing materials from approved, reliable suppliers helps maintain product integrity and supports GMP compliance. This strategic approach minimizes risks tied to substandard inputs.
- Customer service: Responsive customer service teams play a vital role in handling feedback and complaints, which is integral to quality management. Addressing issues quickly not only satisfies customers but also helps identify areas for improvement.
Read More: The 8 Best MRP Software on the Market in the Philippines (2025)
GMP Training
GMP training is essential for helping employees fully grasp what GMP stands for and how it impacts their daily work. By understanding good manufacturing practices, staff become proactive in minimizing risks across all stages of production, from food to cosmetics and pharmaceutical goods.
These training programs typically cover a wide range of topics, including quality control, risk management, and compliance with GMP guidelines. Such comprehensive learning ensures your team can uphold the highest standards, boosting both product safety and customer trust.
Investing in GMP training also strengthens your business’s ability to meet local and international regulations, especially important in the good manufacturing practices food industry. Well-trained employees not only protect your company’s reputation but also support long-term growth by consistently delivering quality products.
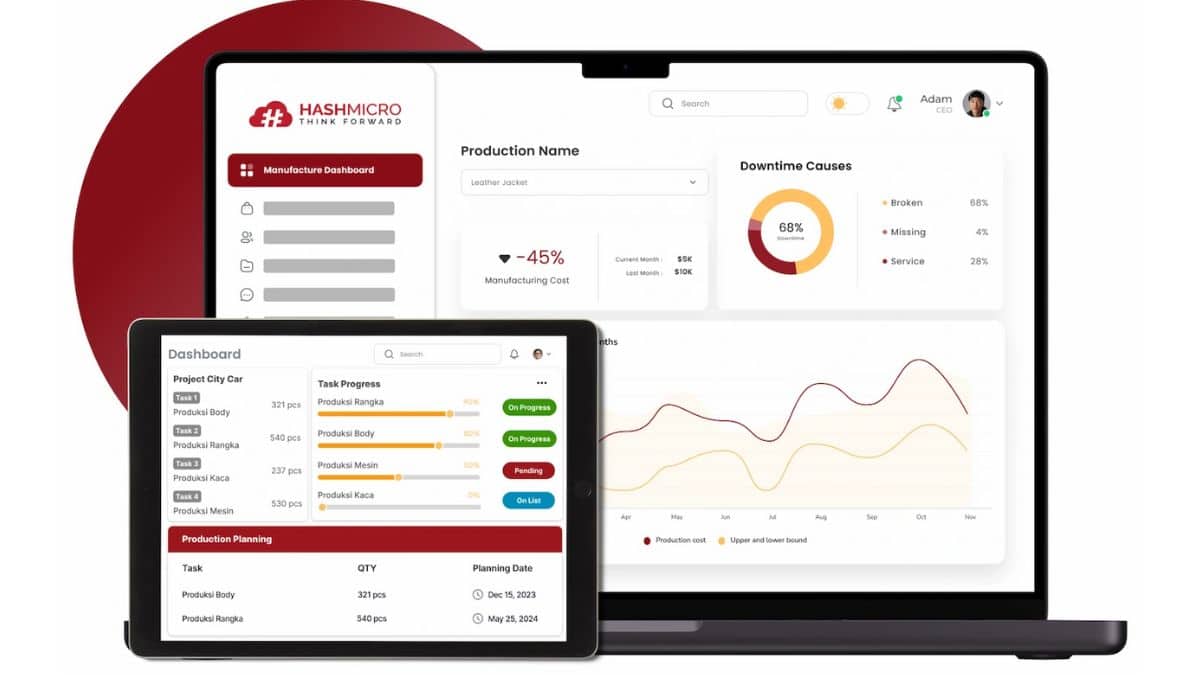
Streamline Good Manufacturing Practices with HashMicro’s Innovative Software
HashMicro’s Manufacturing Software provides a powerful, integrated solution to streamline GMP-related processes accurately and efficiently. Designed to support informed decision-making, this intelligent system ensures that every production activity, quality check, and audit trail is managed with complete transparency and control.
With advanced features, businesses can eliminate manual errors, reduce operational bottlenecks, and ensure strict compliance with GMP guidelines. A free demo will help you explore how a modern manufacturing system can simplify complex workflows and maintain high standards.
Why choose HashMicro? Unlike traditional manufacturing management tools, HashMicro’s Manufacturing Software offers full cross-module integration, customizable reports, and intelligent automation—all tailored to uphold good manufacturing practices across various industries and scales.
Key features of HashMicro Manufacturing Software:
- Manufacturing Quality Control: By automating inspections and checkpoints at each production stage, the system ensures processes consistently meet GMP guidelines. This minimizes defects and protects your brand reputation by delivering safe, compliant products.
- Material, Labor & Overhead Costs Management: It tracks real-time spending on materials, labor, and overhead tied to every batch. This allows precise cost control and supports transparent GMP documentation, which is essential during audits.
- OEE Tracking: Monitoring Overall Equipment Effectiveness highlights equipment bottlenecks and downtime. As a result, you can quickly take corrective actions, safeguarding production quality and maintaining GMP standards.
- Multi-level BoM (Bill of Materials): The system manages complex, tiered BoMs, mapping every raw material back to its source. This strengthens traceability and ensures production follows validated, compliant formulations.
- Real-Time Stock Input and Output for Production: Automatically recording inventory movements provides up-to-date stock levels and audit trails. This helps prevent errors and supports strict GMP recordkeeping requirements.
- Production Order & Work Order Tracking: Capturing each step in production and work orders creates a full digital history of your manufacturing process. This ensures traceability, simplifies compliance checks, and provides reassurance to regulatory bodies.
- Manufacturing Gantt Chart Schedule Management: Visual Gantt charts coordinate production timelines and dependencies. This leads to smoother operations and ensures that processes meet validation plans, which are critical under GMP.
- Manufacturing Cost Actualization: Calculating actual costs per batch after production reveals true profitability. This transparency not only aids GMP-compliant batch records but also drives smarter financial decisions.
- Finished Goods Production Simulation: Simulating production before actual runs uncovers potential risks and inefficiencies. This proactive approach reduces costly rework and supports safer, compliant manufacturing.
- In-Depth Reporting on Time, Materials, Finished Goods: Detailed reports break down time spent, materials consumed, and outputs achieved. This enhances accountability, streamlines GMP audits, and drives continuous improvement of the process.
HashMicro’s Manufacturing Software is designed to adapt and scale with your business, providing you with the clarity and confidence to meet GMP standards strategically. Ready to transform your manufacturing operations? Schedule your free demo today and experience more intelligent, GMP-compliant production with HashMicro.
Conclusion
Embracing Good Manufacturing Practices (GMP) is not just about compliance; it’s a strategic move to protect your products, customers, and business reputation. By adhering to GMP guidelines, companies in the Philippines can minimize risks and build stronger consumer trust.
To help you implement these standards seamlessly, HashMicro offers advanced manufacturing software that automates quality checks, tracks production in real time, and ensures every process aligns with GMP guidelines. With HashMicro, you can confidently reduce errors, maintain consistent quality, and focus on growing your business.
Take the next step by booking a free demo today and see firsthand how our solution can transform your operations. Discover how easy it is to elevate your GMP practices and secure your competitive edge in the market.